COVID-19 Vaccine Update
August 3rd UPDATE:
Vaccination for Children and Teens
The CDC recommends COVID-19 Vaccines for everyone 6 months and older and boosters for everyone 5 years and older, if eligible. Use CDC’s COVID-19 booster tool to learn if and when your child or teen can get boosters to stay up-to-date with their COVID-19 vaccines.
COVID-19 Pfizer Vaccine is NOW AVAILABLE for children 6 months – 4 years old!
This vaccine dosage is based on the age of your child on the day they receive their vaccine, not on the size or weight. Children in this age group will get a smaller dose of the COVID-19 vaccine than teens and adults.
Schedule your child’s vaccine appointment at any of our clinics today. Call/Text 509-488-5256


-------------------------------------------------------------------------------------------------------------------------------
APRIL 4th UPDATE:
Second COVID-19 Booster Shot Available!
The second booster shot is NOW AVAILABLE to people over the age of 50 and certain immunocompromised individuals who received their last booster shot 4 months ago.
A second booster shot of COVID-19 vaccine (Pfizer or Moderna) may be administered to individuals 50 years of age and older at least 4 months after receiving the first booster of any authorized or approved COVID-19 vaccine.
A second booster shot of Pfizer COVID-19 vaccine may be administered to individuals 12 years and older (with certain kinds of immunocompromised disease) at least 4 months after receiving the first booster of any authorized or approved COVID-19 vaccine. These are people who have undergone solid organ transplant, OR who are living with conditions that are considered to have an equivalent level of immunocompromised.
A second booster shot of the Moderna COVID-19 vaccine may be administered at least 4 months after the first booster dose of any authorized or approved COVID-19 vaccine to individuals 18 years and older with the same certain kinds of immunocompromised.
Adults who received a primary vaccine and booster shot from Johnson & Johnson’s Janssen COVID-19 vaccine at least 4 months ago, may now receive a second booster shot.
Call and make your appointment today at 509.488.5256





There is currently a statewide COVID-19 test shortage. At this moment we are providing COVID-19 tests for people with medical necessities.
We CAN test you if:
You have symptoms or are sick
You have been exposed to someone who was positive with COVID-19
We CAN NOT test you if:
You need documentation proof for work/traveling/event
Other options:
You can take your own at home self-test, but may not provide any documented proof of your results.
To help health organizations catch up on their inventory, we ask that you use an at-home self-test if you have some available to you.
At-Home Self-Test
COVID-19 self-tests (also referred to as home tests or over-the-counter (OTC) tests) are one of many risk-reduction measures, along with vaccination, masking, and physical distancing, that protect you and others by reducing the chances of spreading SARS-CoV-2, the virus that causes COVID-19.
Self-tests can be taken at home or anywhere, are easy to use, and produce rapid results.
You can use self-tests, regardless of vaccination status, or whether or not you have symptoms.
Follow all of the manufacturer’s instructions for performing the test.
Consider using a self-test before joining indoor gatherings with others who are not in your household.
If you test negative:
A negative self-test result means that the test did not detect the virus and you may not have an infection, but it does not rule out infection. Repeating the test within a few days, with at least 24 hours between tests, will increase the confidence that you are not infected.
If you test positive:
A positive self-test result means that the test detected the virus, and you are very likely to have an infection and should stay home or isolate for 10 days, wear a mask if you could have contact with others, and avoid indoor gatherings to reduce the risk of spreading disease to someone else.
Isolate yourself from other and inform your healthcare provider, as well as any close contacts.
You can schedule a Telehealth appointment with your provider, where they can assess you and give you medical advice on what you should do next.
When to Consider Self-Testing
Self-tests may be used if you have COVID-19 symptoms or have been exposed or potentially exposed to an individual with COVID-19.
A positive test result indicates that you likely have a current infection, and you should isolate and inform close contacts.
A negative test result indicates that you may not be infected and may be at low risk of spreading disease to others, though it does not rule out an infection. Repeating the test will increase the confidence that you are not infected. Performing serial tests, meaning two or more tests over several days with at least 24 hours between tests—with one test as close as possible to the event you will attend—improves the reliability of testing and reduces your risk of transmitting disease to others even further. Some self-tests require this type of repeat testing in the manufacturer’s instructions.
How to store and use your at-home self-test
Store all test components according to the manufacturer’s instructions until ready for use.
Check the expiration date. Do not use expired tests or test components that are damaged or appear discolored based on the manufacturer’s instructions.
Clean the countertop, table, or other surfaces where you will do the test.
Do not open test devices or other test components until you are ready to start the testing process.
Have a timer ready because you may need to time several of the test steps.
Read test results only within the amount of time specified in the manufacturer’s instructions. A result read before or after the specified timeframe may be incorrect.
Don’t reuse test devices or other components.
After you have the results, discard the specimen collection swab or tube and test in the trash, clean all surfaces that the specimen may have touched, and wash your hands.
If Your Result Shows Invalid or Error
Sometimes invalid results or an error can show on the test device. Invalid results or an error can occur for many reasons. Your specimen may not have been collected correctly, or the test may have malfunctioned.
Invalid test results are rare but can occur. If the self-test shows an invalid result or a test error, the test did not work properly. If this happens, refer to the instructions for use in the package insert and contact the manufacturer for assistance.
Th CDC expanded the recommendation for a booster dose of COVID-19 vaccine to everyone 12 years and older. A booster shot will help increase protection against COVID-19 and variants.
The Western State Scientific Safety Review Workgroup also voted to recommend booster doses for everyone 12 years and older.
Earlier this week, CDC and the Food and Drug Administration (FDA) also made two other changes to their vaccine recommendations:
- People who received the Pfizer-BioNTech COVID-19 vaccine for their primary series should get a booster dose at least 5 months after completing the series. Booster doses were previously recommended at 6 months.
- Children ages 5 to 11 who are moderately to severely immunocompromised should get an additional primary series dose of the Pfizer-BioNTech COVID-19 vaccine 28 days after their second dose.
The shift to 5 months is for Pfizer only. Booster intervals after primary series of Moderna (6 months) and Johnson & Johnson (2 months) have not changed.

There is constantly new information coming out about the COVID-19 vaccine every day! And we want to make sure that it’s easy for you to understand, so you don’t miss out on your vaccinations.
To make it simple:
- Children 5-11 years old can now receive the COVID-19 vaccine
- The Booster Shot is NOW AVAILABLE for ANYONE 18 years of age or older



PFIZER/MODERNA BOOSTER - At least 6 months after your second shot.
65 years or older
18+ in a long term care setting

18+ with underlying medical conditions
18+ work or live in high-risk settings
JOHNSON & JOHNSON - At least 2 months after your last shot.
18 years or older
HIGH - RISH SETTINGS -
First responders (e.g., healthcare workers,
firefighters, police, congregate care staff)
Education staff (e.g., teachers, support staff,daycare workers)
Food and agriculture workers

Corrections workers
U.S. Postal Service workers
Public transit workers
Grocery store workers
If you have any questions on the COVID-19 vaccines or would like to get yours scheduled, call or text us! 509-488-5256
____________________________________
SEPTEMBER 29TH UPDATE:

We have been listening to your questions about the COVID-19 Vaccine 3rd booster shot, so we created a guide to provide you with some answers. Here is a simple way to know whether you need the booster shot or not!
Make sure to follow the recommendations from the COVID-19 Vaccine that you received the first dose from.
Moderna/Pfizer – recommends booster for immunocompromised groups
Receiving active treatment for cancer OR active treatment with high-dose corticosteroids or other drugs that may suppress your immune system

Received an organ transplant OR stem cell transplant in the last two years
Advanced or untreated HIV infection
Moderate or severe primary immunodeficiency
Pfizer – recommends booster 6 months after their Pfizer primary series for immunocompromised group OR the following:
People 65 years and older
Residents in long term care
People 50 to 64 with underlying medical conditions
 People 18 to 49 who are at high risk for severe COVID-19
People 18 to 49 who are at high risk for severe COVID-19
18 to 64 who are at increased risk for COVID-19 exposure
Janssen - No boosters recommended
To schedule an appointment to receive the booster shot, call or text 509-488-5256
_____________________________________
AUGUST 8TH UPDATE:
COVID-19 DELTA VARIANT
Due to rapidly increasing spread of COVID-19 in Washington state driven by the Delta variant, Region 7 public health officers are recommending a change to masking practices:
All residents are advised towear a mask in indoor public places and practicephysical distancing whenever possible.
Health Officers also advise that everyone:
- Increase hygiene measures (handwashing & disinfecting common surfaces)
- Get tested if you develop any potential covid-19 symptoms and isolate yourself until you know the results
- If you have symptoms of covid-19, please call the medical facility before seeking treatment.
Like an “amber alert” or storm warning advisory, regional health jurisdictions are releasing this advisory to keep you, your family, your friends, and your neighbors safe.
Our region is seeing high levels of COVID-19 transmission including the Delta variant. Delta is the name for the B.1.617.2. variant, a SARS-CoV-2 mutation, and it is dramatically more infectious than prior strains. Current data estimates the Delta variant could be more than twice as transmissible as the original strain of SARS-CoV-2.
Delta has been so successful in transmission because those infected with it produce far more virus than those infected with the original version of SARS-CoV-2, making it very easy to spread.
COVID-19 vaccines are extremely effective against the Delta variant’s severity. The risk of becoming severely sick from an infection with the Delta variant is very low for vaccinated individuals who are not immune compromised.
Please, get vaccinated. Un-vaccinated individuals are at higher risk of becoming infected and transmitting the virus to others.
Recommendations if you are not vaccinated:
- Wear a mask indoors in public spaces
- Consider working from home if that is an option for you
- Asymptomatic transmission is high, so please be cautious in gatherings
- Avoid gyms and indoor fitness centers and opt for outdoor exercises like hiking or biking
- Avoid “dining in” and opt for safer alternatives like takeout or a picnic at the park
- Avoid close contact with children who are not a part of your immediate household
- Avoid contact with immune-compromised and high-risk individuals, even those who are vaccinated
Recommendations if you are vaccinated:
- Wear a mask indoors in public space
- Consider working from home if that is an option for you
- Avoid contact with immune-compromised and high-risk individuals, even those who are vaccinated
On Tuesday, July 27, the Centers for Disease Control and Prevention (CDC) issued an Interim Public Health Recommendation for Fully Vaccinated People. Read CDC’s full statement here.
_____________________________________
MAY 24TH UPDATE:
Join us at our Wahluke Clinic, May 26th from 5:30pm – 7pm.
We will only be offering the Pfizer COVID-19 vaccine for everyone, 12 and older, with no restrictions.
Give us a call TODAY to schedule your appointment at 509-488-5256.


_____________________________________
MAY 13TH UPDATE:
CBHA will be offering Pfizer COVID-19 vaccinations starting Thursday, May 20th. The vaccine is now available for ages 12 years and older.
"Today, I adopted CDC’s Advisory Committee on Immunization Practices’ (ACIP) recommendation that endorsed the safety and effectiveness of the Pfizer-BioNTech COVID-19 vaccine and its use in 12- through 15-year-old adolescents. CDC now recommends that this vaccine be used among this population, and providers may begin vaccinating them right away." To continue reading click here.
Read more about the Pfizer Vaccine here.
_____________________________________
APRIL 29TH UPDATE:
The Advisory Committee on Immunization Practices (ACIP) voted on April 23rd to reaffirm its recommendation of the Johnson & Johnson COVID-19 vaccine for persons 18 years of age and older in the U.S population under the Emergency Use Authorization.
A warning statement and an information sheet from Johnson & Johnson will be given to patients at vaccination informing those about the increased risk of thrombocytopenia syndrome.
If you would like to choose another authorized COVID-19 Vaccine, like Pfizer or Moderna, you will be able to do so at CBHA
STATEMENT FROM WA DEPARTMENT OF HEALTH:
"After pausing the use of Johnson & Johnson (Janssen) COVID-19 vaccines on April 13, the Advisory Committee on Immunization Practices (ACIP) met on April 23 for further review of data involving six reported U.S. cases (at the time) of a rare type of blood clot in individuals after receiving the vaccine.
In these cases, a blood clot in the brain formed called thrombosis. This is coupled with low blood platelets, known as thrombocytopenia. When those both occur after a vaccine it is referred to as thrombosis with thrombocytopenia syndrome, or TTS.
The ACIP voted today to reaffirm its recommendation of the Johnson & Johnson COVID-19 vaccine for persons 18 years of age and older in the U.S population under the Emergency Use Authorization. ACIP recommended that the Food & Drug Administration (FDA) include a warning statement and for Johnson & Johnson to include an information sheet at vaccination that informs individuals about the increased risk of TTS. Those concerned about the increased risk may consider choosing another COVID-19 vaccine authorized for use, like the Pfizer or Moderna vaccines.
The Johnson & Johnson vaccine is authorized for use again in Washington state starting April 24, 2021."
CLICK HERE to read more about the temporary halt in administering the Johnson & Johsnon vaccine.
_____________________________________
APRIL 13TH UPDATE:
The CDC has recommended that a pause be put on administering the Johnson & Johnson Janssen COVID-19 Vaccine.
If you are scheduled to receive this vaccine, you will be offered the Moderna COVID-19 Vaccine. If you wish to wait to receive the Janssen vaccine, you will be placed on a waiting list and notified when it becomes available.
People who have received the Johnson & Johnson vaccine who develop a severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination should contact their health care provider.
STATEMENT FROM THE CDC:
"As of April 12, more than 6.8 million doses of the Johnson & Johnson (Janssen) vaccine have been administered in the U.S. CDC and FDA are reviewing data involving six reported U.S. cases of a rare and severe type of blood clot in individuals after receiving the J&J vaccine. In these cases, a type of blood clot called cerebral venous sinus thrombosis (CVST) was seen in combination with low levels of blood platelets (thrombocytopenia). All six cases occurred among women between the ages of 18 and 48, and symptoms occurred 6 to 13 days after vaccination. Treatment of this specific type of blood clot is different from the treatment that might typically be administered. Usually, an anticoagulant drug called heparin is used to treat blood clots. In this setting, administration of heparin may be dangerous, and alternative treatments need to be given."
CLICK HERE to continue reading.

MARCH 17TH UPDATE:
Beginning March 17th, Washington State has moved into Phase 1B Tier 2 of the distribution of COVID-19 Vaccinations.
If you are someone who falls into Phase 1B Tier 2 or a phase that has already begun, and have not gotten your vaccination and would like too, please give us a call so we can schedule your appointment.
If you have any questions regarding the COVID-19 Vaccine, text us at 509-488-5256.





_____________________________________
FEBRUARY 19TH UPDATE:
Currently, we are experiencing a delay in our shipment of COVID-19 Vaccines due to weather. This is a statewide issue and there is no tentative date of when they will arrive to our facility.
If you are due for your second COVID-19 vaccine dose, don’t worry! We put together some recommended information from the CDC if there is a delay on receiving your vaccine.
2nd dose should not be scheduled to be received earlier than the recommended 1 month (28 days)
2nd dose can be administered within a grace period of 4 days earlier than the recommended date for the 2nd dose is still considered valid.
Doses inadvertently administered earlier than the grace period should not be repeated.
The 2nd dose should be administered as close to the recommended interval as possible.
If it is not feasible to adhere to the recommended interval and a delay in vaccination is unavoidable, the 2nd dose of Moderna COVID-19 vaccines may be administered up to 6 weeks (42 days) after the 1st dose.
There are currently limited data on efficacy of mRNA COVID-19 vaccines administered beyond this window. If the 2nd dose is administered beyond these intervals, there is no need to restart the series.
Thank you for staying patient in this process and for doing your part and getting vaccinated against COVID-19. If you are interesting in getting vaccinated, give us a call to be put on our waitlist.
If you have any questions about the COVID-19 Vaccine, call us or text us at 509-488-5256



_____________________________________
JANUARY 27TH UPDATE:
COVID-19 Vaccinations are still underway for those under Phase 1B, Tier 1!
What does this mean?
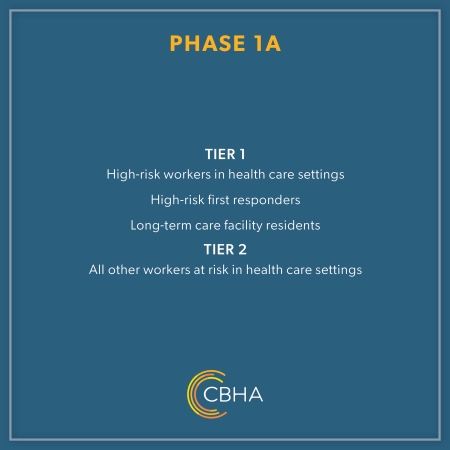
Phase 1B, Tier 1 is for individuals that are:
• 65 or Older
• 50 or Older and live in a multigenerational household
If you are an individual that falls into Phase 1A and have not yet received your vaccine, you are still able to do so.
ROYAL CITY: We will be having a COVID-19 Vaccine Clinic in Royal City on Wednesday, February 3rd from 10am – 2pm at the New Life Church.
Call/text to schedule your appointment (while supplies last)! 509-488-5256
You do not need to be a CBHA patient to receive the vaccine. Please be sure to bring your ID.
If you can’t travel to Royal City and would like to be placed on a waiting list in our Othello, Connell or Mattawa Clinics, please give us a call.
What does multigenerational household mean?
A Household where individuals from 2 or more generations live in such as an elder and a grandchild.
_____________________________________
JANUARY 19TH UPDATE:
The COVID-19 Vaccine is NOW AVAILABLE for individuals in Phase 1B Tier 1!
All individuals that are eligible in Phase 1A and Phase 1B Tier 1 will be able to receive the vaccine. (65 and older and/or 50 and older and living in a multigenerational household (two or more generations).
We will be having a COVID-19 Vaccine Clinic at our Wahluke Clinic this Saturday, January 23rd from 10am-3pm for those who qualify under the guidelines of the current phase. You do not need to be a CBHA patient to receive the vaccine, simply call/text to schedule your appointment or arrive as a walk-in (while supplies last). Please be sure to bring your ID.
If you can’t travel to Mattawa and would like to be placed on a waiting list in our Othello or Connell Clinics, please give us a call. 509-488-5256.
What does multigenerational household mean?
A household where individuals from 2 or more generations live such as an elder and a grandchild.
_____________________________________
DECEMBER 18TH UPDATE:
The COVID-19 Vaccine has received Emergency Use Authorization approval by the Food and Drug Administration and the first supplies are in Washington!
There are 4 phases to how the vaccine will be delivered, and the Center of Disease Control is directing that the first dose of vaccines be given to high-risk healthcare workers.
Because the vaccinations are currently limited, we will not be administering them to the public just yet. We will keep you updated on when the vaccine will be available for everyone.
_____________________________________
Learn about the COVID-19 Vaccine:
How COVID-19 Vaccines Are Made (VIDEO)
How Would COVID-19 Vaccines Work In Your Body (VIDEO)
COVID-19 Vaccine (VIDEO)
How are COVID vaccines being produced faster?
Usually vaccine testing and production are done as separate steps, but because of the pandemic, vaccines are being developed on parallel tracks - meaning we’re still doing both steps, just at the same time.
How are the vaccines being tested?
Several different COVID vaccines are in testing right now. Each of them goes through more than one clinical trial; first with a small group of volunteers, then a couple hundred, then thousands.
Find answers to frequently asked questions and get the latest information at CovidVaccineWA.org
Will you have to pay for the vaccine?
No, the vaccine is free for you. This is true for people who have private insurance, Medicaid, Medicare, or are uninsured. Please be sure to bring your insurance card with you.
If you do not have insurance, let us know and our Patient Benefits team can help you secure coverage.